Executive Summary
Arsenic is the 53rd most abundant element on Earth [1]. Frequently known for its toxicity and use as a poison, arsenic and its affiliated compounds have a wide array of applications, from the semiconductor industry to the preservation of wood. Arsenic has also been used for thousands of years in traditional Chinese and Indian medicines [1,2].
As a metalloid, arsenic is both metallic in nature while simultaneously sharing characteristics of the group 15 nonmetals nitrogen and phosphorus [3,4]. Arsenic most typically bonds with a +3 or +5 oxidation state, and easily binds to oxygen and sulfur. Arsenic is also capable of bonding with Group 1 and 2 elements, forming near-ionic Zintl phases [1].
In nature, arsenic is frequently found in sulfidic ores and in minerals with the general formula MAsS [1]. Through the burning of fossil fuels and the smelting of sulfidic ores, notably copper, humans are the greatest contributors of arsenic to the atmosphere and to the water. This can have severe adverse environmental effects [1,2,5].
Recently, arsenic trioxide (As2O3) has replaced traditional forms of chemotherapy as a treatment for promyelocytic leukemia (PML) [2]. Future research aims to treat many other forms of cancer with arsenic, perhaps via the use of nanobins [2].
Contents
1 Historical Use
2 Environmental and Anthropogenic Sources
3 Elemental Chemistry
4 Toxicity
4.1 Arsenic Acid
4.2 Arsenous Acid
5 Arsenic as a Therapeutic
5.1 PML
5.2 Current Research
1 Historical Use
Colloquially referred to as the “King of Poisons and the Poison of Kings”, arsenic is well known for its role in the poisonings of kings, dukes, emperors, and even fictional characters. Notable people who were assassinated by arsenic are Francesco de’Medici (Grand Duke of Tuscany), King George III of the UK, the Guangxu Emperor of China, and King Faisal I of Iraq. Despite its well known use as a poison, arsenic and its affiliated compounds have a diverse array of historical and current applications.
Medically, arsenic compounds have been used for thousands of years. In traditional Chinese medicines, arsenic was used as a devitalizing agent in the treatment of root canals [2]. The arsenic compounds (Figure 1) realgar (As4S4) and orpiment (As2S3) were also crushed and used in the treatment of various chronic skin conditions, respiratory conditions, ulcers, and parasitic infections [2]. The ancient Greeks also frequently used arsenic in the treatment of certain chronic conditions. Hippocrates and other ancient Greek physicians used arsenic as an escharotic, a substance that could be applied to skin and breast cancers to generate a necrotized black scab [2].

Figure 1. Arsenic sulfide minerals from Nevada, U.S.A. The orange-colored mineral is orpiment (As2S3) while the red-colored mineral is realgar (As4S4). Image from Dakota Matrix Minerals (https://www.dakotamatrix.com/)
Arsenic became popularized in Western medicine in the late 18th century with the invention of a solution of potassium arsenite (KAsO2). Patented under the name “Fowler’s Solution” after the English physician Thomas Fowler who coined its use as a treatment of intermittent fever, Fowler’s Solution became somewhat of a panacea for hundreds of conditions, including eczema, leukemia, epilepsy, hysteria, ulcers, heart palpitations, anemia, and asthma [1,2]. The use of Fowler’s solution continued into the early 20th century. In 1910, Paul Ehrlich, a German physician and the founder of chemotherapy, was experimenting with the synthesis of medically active organic arsenicals and discovered the compound arsphenamine [1,2]. The compound, patented under the name Salvarsan, became the standard treatment for syphilis until penicillin became widely available during World War II [1,2].
Arsenic compounds have served a variety of non-medicinal uses. Its salts were frequently used as dyes, such as Paris Green (copper(II) acetoarsenite). Other salts have been used as pesticides and insecticides (particularly in viticulture), and as wood preservatives [1,2]. During the plague, arsenic was frequently used as a rodenticide. Arsenic has also played a role in chemical warfare. In WWI, organic arsenide compounds were used by the German army as chemical weapons. The United States frequently used Agent Blue during the Vietnam war as a deforestation agent [1].
Metallic arsenic is frequently used in industrial technology [1]. Alloys of lead containing arsenic are often used in storage batteries. Automotive solder often contains small quantities of arsenic. Gallium arsenide is one of the most commonly used semiconductive materials in technology, second only to silicon. While circuits made from GaAs are faster, they are significantly more expensive and their disposal is more complex [1].
2 Environmental and Anthropogenic Sources
Arsenic is the 53rd most abundant element and occurs naturally in sulfidic ores (Cu, Ni, Zn, Co, Pb). The arsenic sulfides (Figure 1) realgar (As4S4) and orpiment (As2S3) along with arsenopyrite (FeAsS) are some of the best known arsenic-containing minerals [1,2]. Due to its flexible bonding chemistry, hundreds of arsenic-containing minerals exist [1].
Humans are the greatest contributors of arsenic to the environment. The smelting of sulfidic ores (particularly copper ores) releases gaseous arsenic trioxide (As2O3) into the air [1]. Elemental arsenic can be acquired via condensation of this gas followed by another round of smelting [1]. Most refinement operations in Europe and the United States have closed due to environmental concerns, leaving China as the world’s top producer. The burning of fossil fuels also tends to release particulate arsenic compounds into the atmosphere [1].
3 Elemental Chemistry
Element number thirty-three, arsenic is a member of the Group 15 elements (nitrogen, phosphorus, antimony and bismuth). Group 15 elements share a common valence shell of five electrons, s2p3, giving rise to primarily (III) and (V) species. As a whole, Group 15 elements are diverse in metallic character, melting/boiling point, density, and crystalline geometry (Table 1). However, most compounds containing Group 15 elements are very stable, contribution to their widely recognized inert and/or toxic character.
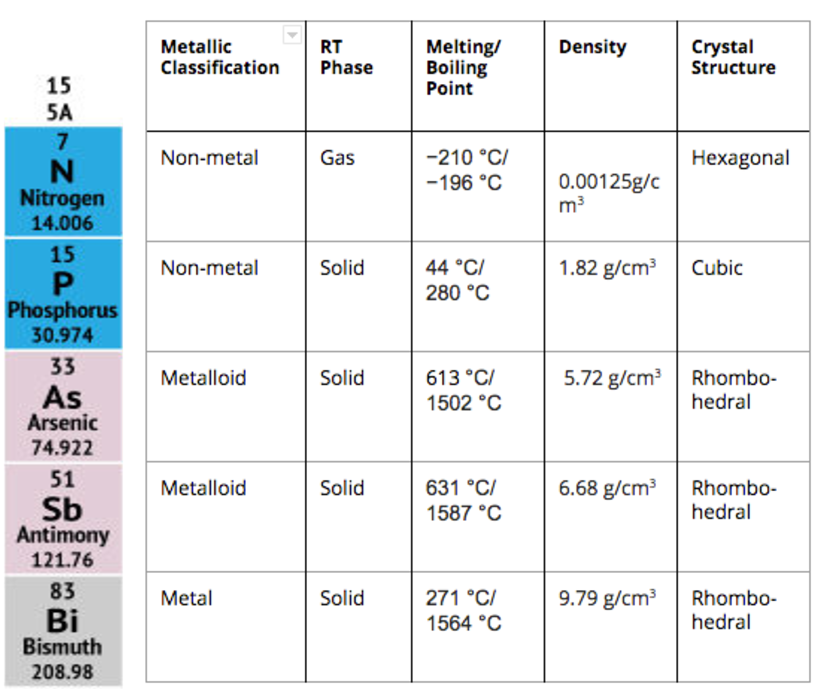
Table 1. Properties of Group 15 Elements.
Reflective of it’s classification as a metalloid, arsenic’s chemical properties cannot be exclusively labeled as having metal or non-metal character (Table 1). Regarding physical properties, arsenic is more similar to antimony and bismuth, sharing a high melting/boiling point, high density, and rhombohedral crystalline geometry. However, considering bonding, arsenic is more comparable to phosphorus, displaying covalent character. Arsenic is naturally found as As(I), As(III) or As(V) and can adopt a coordination number in the range of 3-6.
4 Toxicity
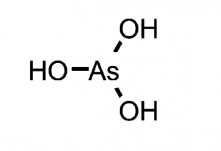
Figure 1A. Structure of Arsenous Acid

Figure 1B. Structure of Arsenic Acid
Arsenic exposure is toxic to the human body. If exposure is brief and of a high concentration, acute arsenic poisoning leads to vomiting, diarrhea, bloody urine (liver/kidney failure), heart failure, and eventually death [2]. Chronic arsenic poisoning is most frequently caused by groundwater contamination, but also from human activities such as mining, smelting, and agricultural runoff [7]. Groundwater contamination with Arsenic is particularly a problem in Bangladesh, East Asia, South America, and the Western United States [7]. The first symptom of chronic arsenic exposure is the hyperpigmentation of skin (when your skin becomes darker than the surrounding skin) [7]. Exposure levels to develop symptoms are 0.5 to 3 years of contamination levels 0.04mg/kg/day or 5-15 years of 0.01mg/kg/day can cause hyperpigmentation [2]. More severe symptoms include bladder, lung, liver, kidney, and prostate cancers, anemia, neuropathy, encephalopathy, and diabetes [2].
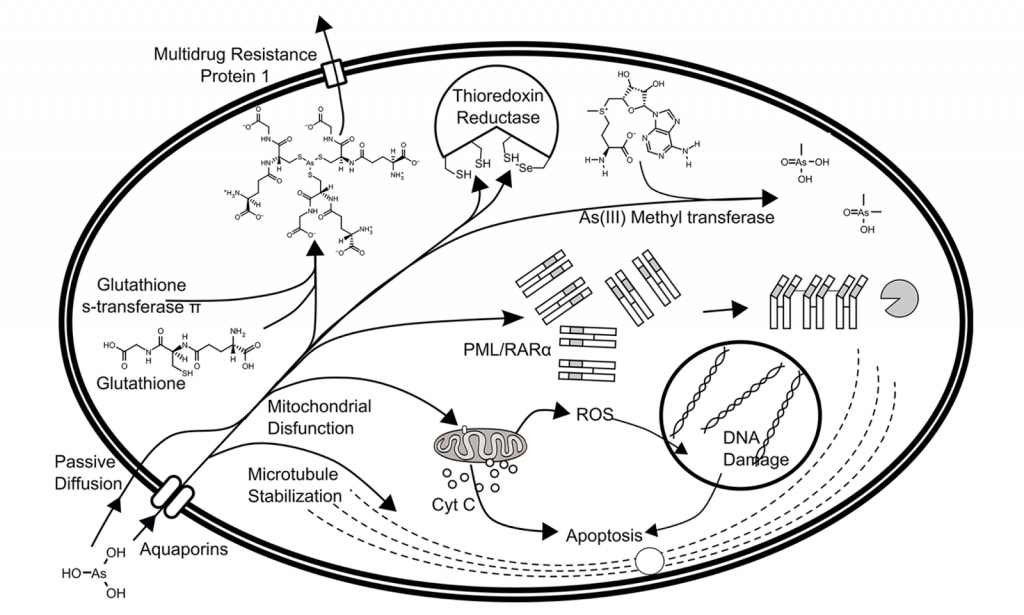
Figure 2. Toxicity of arsenous acid in the human cell. Arsenic trioxide (As2O3) is converted to arsenous acid at physiological pH. Upon entering cells via passive diffusion or via aquaporins, arsenous acid causes oxidative stress, can interfere with phosphorylation reactions, and can disrupt microtubule polymerization/depolymerization. Image from Swindell et al. (2013)
4.1 Arsenic acid
Arsenic is present in the body as two major species. Arsenic (V) Acid is very similar to phosphoric acid and can function as a phosphate analog [2]. It can participate in phosphorylation reactions such as the first step of glycolysis and can function as phosphatase inhibitors, as the intermediate formed with Arsenic is more stable than the native phosphoester intermediate [2].
4.2 Arsenous acid
The second major species present in the body is Arsenous(III) acid. It has its effects on cells by interacting with thiol groups present on Cysteine residues and glutathione (GSH) (Figure 2) [2]. Arsenous acid can enter the cell through passive diffusion or through an aquaporin like transporter (phosphate transporter), aquaglyceroporin 9 (AQP9) [2]. Arsenic is transported out of the cell by its interactions with glutathione, catalyzed by glutathione-S-transferase pi (GST-) and downstream proteins like multidrug resistance export protein 1 (MRP1) [2]. Glutathione (antioxidant) and thioredoxin reductase are two important players that function to keep reactive oxygen species (ROS) levels in check in cells. Excess Arsenic depletes these sources, leading to increased ROS levels [2]. Arsenous acid also interacts with mitochondrial membrane transition pore complex, by targeting the voltage dependent anion channels (VDAC); this causes the collapse of mitochondrial membrane potential and release of cytochrome c from mitochondria into cytoplasm, leading to mitochondrial dysfunction and apoptosis [2]. Arsenous acid also binds to microtubules, stabilizes tubulin, preventing depolymerization thus halts the cell cycle [2]. Arsenic III methyltransferase catalyzes the methylation of arsenous acid via three cysteine residues in an SN2-like fashion. Methylated arsenous acid species has increased reactivity with sulfur ligands, worsening their effects on the cell mentioned above [2].
5 Arsenic as a Therapeutic
5.1 Promyelocytic leukemia treatment (PML)
Arsenic trioxide (AsO3) is the most commonly used treatment for promyelocytic leukemia (PML) [2]. PML is a form of acute leukemia that is deadly within a week if not treated. In >98% of cases, PML is caused by a chromosome translocation between chromosomes 15 and 17, resulting in a fusion of the PML gene with the retinoic acid receptor alpha (RARα) gene. The fusion of these two genes results in the expression of a PML/RARα hybrid protein. PML/RARα binds with enhanced affinity to multiple sites on the cell’s DNA [2]. It also disrupts the formation of nuclear bodies and recruits histone deacetylases. The net effect is an overall transcriptional block, preventing a patient’s white blood cells from properly developing [2].
PML has traditionally been treated with all-trans retinoic acid (ATRA) paired with anthracycline-based chemotherapy (typically danorubicin) [2]. While this therapy results in complete remission rates around 90%, its side effects are frequently intense and disfavorable. In the 1970s, a group of physicians at Harbin Medical University in China studied traditional Chinese medicines for leukemia and identified arsenic as an active ingredient. This led to a multi-year study testing the use of low-dose intravenous As2O3 as a treatment for PML [2]. The results, released in the mid-90s, showed impressive side-effect profiles and complete remission comparable to ATRA therapy. In 2000 the US FDA approved As2O3 as a treatment for PML. In recent years, arsenic trioxide treatment has surpassed ATRA therapy as the most common PML treatment [2].
Mechanistically, As2O3 targets PML cells because the AQP9 acquaglyceroporin is significantly upregulated in these cells [2]. The problematic PML/RARα fusion protein is also a strong chelator of arsenous acid, and it is hypothesized this chelation leads to aggregation and proteasomal degradation of multiple PML/RARα proteins [2]. Altogether, As2O3 treatment selectively poisons cancerous cells. The degradation of PML/RARα leaves functional versions of PML and RARα produced by the non-modified chromosomes, allowing treated cells to be “knocked” into a noncancerous state [2].
5.2 Current Research
While arsenic therapeutics have been highly successful for treating hematological cancers, the bulk of current research aims to improve their efficacy for treating solid tumors. Historically, treatment of solid malignancies has been limited by rapid clearance of therapeutics by immune response mechanisms, specific to arsenic.
For example, Ahn et al. are investigating nanoscale liposomes, termed nanobins, for the stable encapsulation and delivery of As2O3 to solid tumors [6]. Arsenic is loaded into the nanobins via a metal ion gradient, whereby arsenic acid complexes with divalent metal ions (Ni2+, Co2+, and Pt2+) causing precipitation within the liposome, yielding a stable crystalline cargo (Figure 3) [6]. The nanobins have generated As2O3 concentrations greater than 270mM, yet remain less toxic to off-target sites due to the protective presence of the liposomal bilayer [6]. In a mouse model of breast cancer, the nanobins delivered 2-3 times as much arsenic to solid tumors as compared to traditional As2O3 treatment [6].
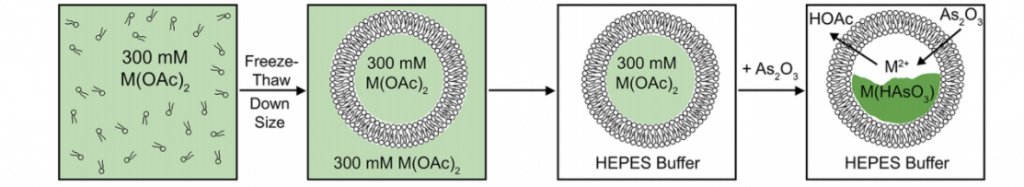
Figure 3. Schematic representation of nanobin assembly. Panel 1 – Lipids and cholesterol are immersed in a metal acetate,M(OAc)2, solution. Panel 2 – Self-assembly of the liposomal bilayer. High-pressure extrusion techniques are used to control nanobin size. Panel 3 – Excess M(OAc)2 is removed and replaced with HEPES buffer. Panel 4 – Arsenic loading is driven by complexation of As2O3 with divalent (M2+) metal ions and the efflux of protonated HOAc. Image from Swindell et al. (2013).
Sources
[1] Crabtree, Robert H. Encyclopedia of Inorganic Chemistry. Arsenic: Inorganic Chemistry. Chichester: Wiley, 2009. Print.
[2] Swindell, E.P., Hankins, P.L., Chen, H. Miodragovic, D.U., and Halloran, T.V. Anticancer Activity of Small-Molecule and Nanoparticulate Arsenic(III) Complexes. Inorganic Chemistry, 2013, 52, 12292-12304.
[3] Nidhubughaill. The Structure and Reactivity of Arsenic Compounds- Biological Activity and Drug Design. Structure and Bonding, 1991, 78, 129-190.
[4] Protasiewicz, J.D. Nitrogen, phosphorus, arsenic, antimony, and bismuth. Annual Reports on the Progress of Chemistry, Section A: Inorganic Chemistry, 2013, 109, 66-79.
[5] Miller, W.H., Schipper, H.M., Lee, J.S., Singer, J., Waxman, S. Mechanisms of Action of Arsenic Trioxide. Cancer Research, 2002, 62, 3893-3903.
[6] Ahn, R.W., Barrett, S.L., Raja, M.R., Jozefik, J.K., et al. Nano-Encapsulation of Arsenic Trioxide Enhances Efficacy against Murine Lymphoma Model while Minimizing Its Impact on Ovarian Reserve In Vitro and In Vivo. PLoS ONE , 2013, 8(3), https://doi.org/10.1371/journal.pone.0058491.
[7] Davis, C.P. Arsenic Poisoning. MedicineNet.com 2016 http://www.medicinenet.com/arsenic_poisoning/article.htm
