Platinum Anticancer Drugs
EXECUTIVE SUMMARY: Since cisplatin, the first FDA approved Pt(II) anticancer drug, went on the market in 1978, platinum anticancer drugs have been a great success. [1] Cisplatin has been especially effective in treating testicular cancer; it increased long-term survival rates of testicular cancer patients from less than 10% to greater than 90%. [1] The anti-cancer mechanism of cisplatin relies on its cis binding two guanine residues in DNA, causing a kink in the chromosome and preventing DNA replication. Because of this cis binding mechanism, the synthesis of cisplatin (vs. transplatin) is highly important. [1] While cisplatin is a highly effective drug in many cases, many cancers eventually develop resistance. It is necessary to think about the possibilities of new types of drug delivery and Pt(IV) drugs. [1]

Structure of cisplatin, the canonical platinum therapeutic.
Cisplatin
Cisplatin is the canonical Pt(II) anticancer drug. [1 ]There are several characteristics that make cisplatin a good anticancer drug. Cisplatin is soluble in the bloodstream and cytoplasm, has a specific targeting mechanism, and a planned ligand design.1 In cisplatin’s ligand design, it is important that it has a neutral charge, square planar geometry, and two sets of cis ligands–two labile and two inert. [1] These sets of ligands allow for more fine-tuning of cisplatin’s properties, including toxicity, solubility, and lipophilicity. [2]
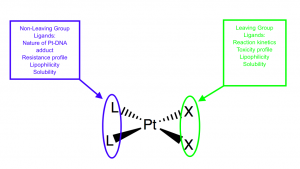
Each set of cis ligands can influence the character of the cisplatin molecule (Adapted from Wilson, et al.) [2].
Binding
Cisplatin binds to DNA, ultimately inhibiting DNA transcription and translation, triggering cell death. [1] Cisplatin prefers to bind to guanines, specifically at the N7 position. [1] Cisplatin binds two adjacent guanines, creating a crosslink that kinks DNA structure. [1] Cisplatin’s ability to bind to adjacent guanines arises from its cis structure, as the cis chlorines can be exchanged for water molecules or for guanines by cisplatin. [1] Cisplatin also prefers to bind on the 5’, rather than the 3’, side of the DNA double helix, potentially because the N7 position is closer to the 5’ side (~3Å) than the 3’ side (~5Å). [3]
Coordination
Pt(II) has an electron configuration of [Xe]4f145d6, which leads to a square planar geometry with four ligands. Pt(II) is a soft acid (atomic radius: 175 pm) and therefore binds preferentially with soft bases like CN- and CO-. However, it is possible to for Pt(II) to bond with borderline or hard ligands, like cisplatin ligands NH3 and Cl-.
Mechanism of Entry into Cell
In examining cisplatin’s entry into the cell, there are three central components: aquation, cellular uptake, and chlorine concentrations. It is known that it is necessary for cisplatin to be aquated at least once in order for it properly bind to DNA. [1] However, it is not known if cisplatin is aquated outside of the cell in the bloodstream or inside the cell in the cytosol. [1] When cisplatin is aquated once, exchanging a single chlorine for a water molecule, it acquires a +1 charge. When cisplatin is aquated twice, exchanging two chlorines for two water molecules, it acquires a +2 charge. If cisplatin is aquated and acquires a charge outside of the cell, it is likely that it enters the cell via OCT1, an organic cation transporter, or CTR1, a copper(I) transporter. [1] Both OCT1 and CTR1 transport charged species and thus would be able to transport a charged cisplatin molecule. If cisplatin is aquated inside the cell, it is likely that it enters the cell via passive diffusion, as the molecule is uncharged. [1]
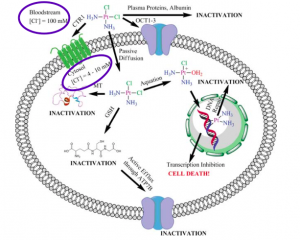 ( Image: Johnstone, et al.)
( Image: Johnstone, et al.)
It is also important to note the chlorine concentrations in the bloodstream and in the cell and their impact on cisplatin. In the bloodstream, the chlorine concentration is about 0.1M. [1] Inside the cell, it is 0.01-0.02M, which is approximately 50 to 100 times less chlorine than in the bloodstream. [1] This information also aids with examining where cisplatin’s aquation occurs. The lower chlorine concentration in the cell favors cisplatin aquation, as that would increase the cell’s chlorine concentration. The bloodstream might be less favorable for cisplatin aquation, as the concentration of chlorine is already relatively high. [1]
Trans Effect and Cisplatin Synthesis
The trans effect is a kinetic effect where ligands influence the lability of the ligand trans to them. It is regarded as being important for transition states, especially for square planar molecules undergoing ligand substitution. Square planar complexes undergo associative ligand substitution, where the incoming and outgoing ligands are both associated with the complex during and therefore creating an electron-dense transition state. This electron density is stabilized by trans ligands with high π acidity (the ability to accept electrons from a metal’s π orbital) and therefore a high trans effect. The trans effect series is as follows:
F−, H2O, OH− < NH3 < py < Cl− < Br− < I−, SCN−, NO2−, SC(NH2)2, Ph− < SO32− < PR3, AsR3, SR2, CH3− < H−, NO, CO, CN−, C2H4,
with molecules at the end of the series having the highest trans effect. [4]
The trans effect is an important consideration in obtaining correct stereochemistry. During cisplatin synthesis, it is necessary to start with [PtCl4]2- because Cl- has a higher trans effect than NH3. As shown in the figure below, the first NH3 will be added to a random position. The second NH3, however, will be added cis to the first NH3 because the Cl- atoms trans to another Cl- are more labile than the Cl- atom trans to the first NH3.
Starting with [Pt(NH3)4]4+ leads to formation of transplatin because the most labile ligand after the first Cl- binds is the NH3 trans to itself.
Trans Influence and Cisplatin Activity
The trans effect should not be confused with the trans influence, which is a thermodynamic phenomenon in which ligands influence the bonding length and bond strength. This effect is determined by the extent to which one ligand pulls electron density toward itself, thus decreasing electron density between the metal and its trans ligand. The trans influence and trans effect do not always correlate with each other. [4] As seen above, for instance, Cl- has a higher trans effect than NH3. NH3, however, has a stronger trans influence than Cl-: crystal structures show that all M-Cl bond lengths are equal in a metal-Cl4 complex, . When an NH3 is substituted trans to a Cl-, however, the M-Cl bond length increases. [5] This indicates that NH3 is more electronegative than Cl- and pulls more electron density through the inductive effect, therefore having a higher trans influence than Cl-. This makes the -Cl ligand more easily exchanged, allowing for guanine to be cross linked.
Resistance and Shortcomings
Cells can develop resistance to cisplatin by binding it with sulfur-containing proteins and molecules like glutathione. [6] Because of the soft base nature and π acidity of these sulfur-containing ligands, these molecules can bind cisplatin with low reversibility and inactivate its DNA binding capability. [4] Some nucleotide excision repair (NER) proteins are also capable of removing bound guanidines and allowing for the repair of crosslinked DNA. Cancer cells with higher expression of these DNA repair proteins are more likely to not respond to cisplatin treatment.
Other downsides of cisplatin include its general toxicity. Cisplatin in vivo localization is poor, and it causes nonspecific cell death throughout the body. Cisplatin is especially toxic to the renal system, causing inflammation and kidney damage. [7]
Pt(IV) Prodrugs
Since cisplatin and associated compounds remain the standard of treatment in cancer therapy, high general toxicity as well as potential for resistant cancer cell lines mean reducing toxicity and increasing efficacy is an important field of research for improving cancer therapy on the whole. [1] The use of platinum (IV) is one proposed idea for reducing off-site cell killing. Pt(IV) can be cossidered a ‘pro-drug’ as it is inactive due to its low-spin d6 outer shell but is possibly readily reduced to the active Pt(II) in the hypoxic, reducing environment of cancer cells.
Platinum (IV) prefers six binding partners in an octahedral geometry with two additional ligands to Pt(II). These axial ligands allow for the drug to me augmented in ways that do not affect the active (II) form. Ligands that can improve the efficacy of treatment, such as ligands conjugated to other anticancer drugs that are effective in equimolar dosages, can be attached, improving therapy. Further, the two additional ligands provide binding sites for vectors that can improve the localization of cisplatin in vivo. Peptides that bind cell surface proteins that are over expressed in cancer cells provide one option for improving targeting. Nanotechnology, such as nanotubes, gold nanoparticles, or polymeric micelles are additional candidates for improving the delivery of other drugs to cancer tissue that could also exploit Pt(IV). [1]
See Also
This video shows an animation of cisplatin interacting with DNA. It specifically provides a valuable visual of cisplatin binding to the guanines of DNA.
References
- Johnstone, T.C.; Wilson, J.J.; Lippard, S.J. Monofunctional and Higher-Valent Platinum Anticancer Agents. Inorg. Chem. 2013, 52, 12234-12249 dx.doi.org/10.1021/ic400538c.
- Wilson, J.J.; Lippard, S.J. Synthetic Methods for the Preparation of Platinum Anticancer Complexes. Chem. Rev. 2014, 114, 4470-4495 dx.doi.org/10.1021/cr40043141.
- Mantri, Y.; Lippard, S. J.; Baik, M. Bifunctional Binding of Cisplatin to DNA: Why Does Cisplatin Form 1,2-Intrastrand Cross-links with AG, But Not with GA? J. Am. Chem. Soc. 2007, 129, 5023-5030 dx.doi.org/10.1021/ja067631z.
- Gray, H.B., Stiefel, E.I., Valentine, J.S., Bertini, I., ed. Biological Inorganic Chemistry: Structure and Reactivity. University Science Books: USA, 2007.
- [Citation needed]
- Kartalou, M. and Essigmann, J.M. (2001). Mechanisms of resistance to cisplatin. Mutat Res-Fund Mol M 478(1):23-43.
- Miller, R.P., et al. (2010). Mechanisms of Cisplatin Nephrotoxicity. Toxins (Basel) 2(11):2490-2518.
